Evaporation: Does heat speed up the evaporation of normal propyl alcohol compared to cold temperatures?
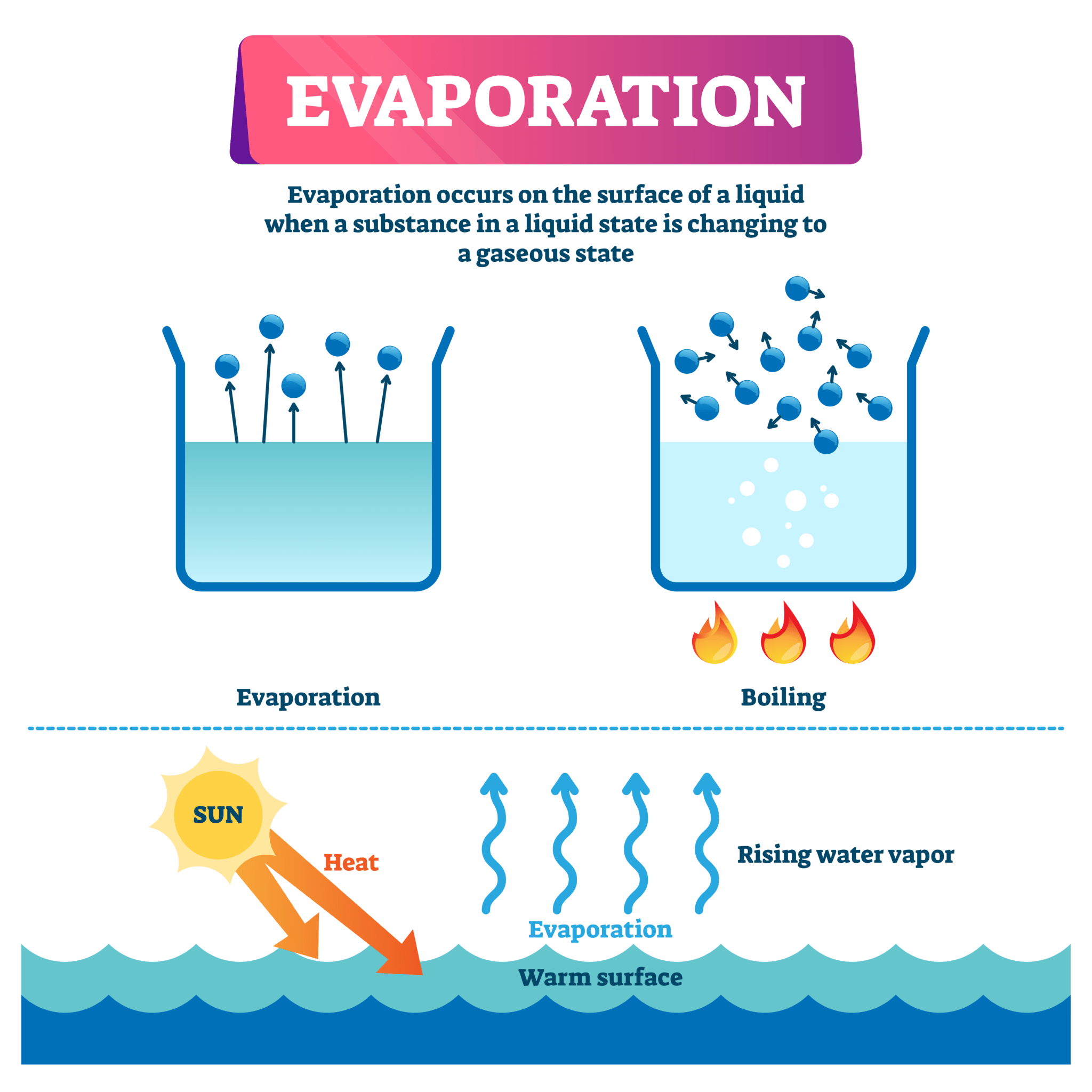
Evaporation is the process by which a liquid changes into a gas. The rate of evaporation is affected by several factors, including temperature. In general, the higher the temperature, the faster the evaporation rate. This is because heat provides the energy needed for molecules to escape from the liquid and enter the gas phase.
In the case of normal propyl alcohol, the evaporation rate is indeed faster at higher temperatures. This is because the molecules of normal propyl alcohol have more energy at higher temperatures, which allows them to escape from the liquid more easily. The rate of evaporation of normal propyl alcohol at 25C is about twice as fast as the rate of evaporation at 0C.
The evaporation of normal propyl alcohol is an important process in many industrial and commercial applications. For example, it is used in the production of paints, varnishes, and other coatings. It is also used as a solvent in the manufacture of plastics, pharmaceuticals, and other products.
The evaporation of normal propyl alcohol can also be a problem in some cases. For example, it can lead to the loss of valuable products during storage or transportation. It can also create a fire hazard if the vapors are allowed to accumulate in a confined space.
Evaporation of Normal Propyl Alcohol
The evaporation of normal propyl alcohol is a process that is affected by temperature. In general, the higher the temperature, the faster the evaporation rate. This is because heat provides the energy needed for molecules to escape from the liquid and enter the gas phase.
- Temperature: The rate of evaporation of normal propyl alcohol is directly proportional to the temperature.
- Surface area: The rate of evaporation is also affected by the surface area of the liquid. The greater the surface area, the faster the evaporation rate.
- Airflow: The rate of evaporation is also affected by the airflow. The greater the airflow, the faster the evaporation rate.
- Humidity: The rate of evaporation is also affected by the humidity of the air. The higher the humidity, the slower the evaporation rate.
- Pressure: The rate of evaporation is also affected by the pressure of the air. The higher the pressure, the slower the evaporation rate.
The evaporation of normal propyl alcohol is an important process in many industrial and commercial applications. For example, it is used in the production of paints, varnishes, and other coatings. It is also used as a solvent in the manufacture of plastics, pharmaceuticals, and other products.
Temperature
The rate of evaporation of a liquid is the rate at which its molecules escape from the liquid and enter the gas phase. The rate of evaporation is affected by a number of factors, including temperature, surface area, and air flow. Temperature is the most important factor affecting the rate of evaporation. The higher the temperature, the faster the rate of evaporation.
This is because temperature is a measure of the average kinetic energy of the molecules in a substance. The higher the temperature, the more kinetic energy the molecules have, and the more likely they are to escape from the liquid and enter the gas phase.
The relationship between temperature and the rate of evaporation is linear. This means that the rate of evaporation increases by a constant amount for each degree that the temperature is increased.
The evaporation of normal propyl alcohol is an important process in many industrial and commercial applications. For example, it is used in the production of paints, varnishes, and other coatings. It is also used as a solvent in the manufacture of plastics, pharmaceuticals, and other products.
The rate of evaporation of normal propyl alcohol is an important factor to consider in these applications. By understanding the relationship between temperature and the rate of evaporation, it is possible to control the rate of evaporation and optimize the performance of these products.
Surface area
The surface area of a liquid is the amount of its surface that is exposed to the air. The greater the surface area, the more molecules of the liquid are able to escape into the gas phase, and the faster the evaporation rate. This is because the molecules at the surface of the liquid have more energy than the molecules in the interior of the liquid, and they are therefore more likely to escape.
The evaporation of normal propyl alcohol is a good example of how surface area affects the rate of evaporation. If you have a small amount of normal propyl alcohol in a wide, shallow dish, it will evaporate more quickly than if you have the same amount of normal propyl alcohol in a tall, narrow container. This is because the surface area of the liquid is greater in the wide, shallow dish, so there are more molecules of the liquid that are able to escape into the gas phase.
The surface area of a liquid is an important factor to consider in many industrial and commercial applications. For example, in the production of paints and coatings, the surface area of the liquid is a key factor in determining the drying time. By understanding the relationship between surface area and the rate of evaporation, it is possible to control the drying time and optimize the performance of these products.
Airflow
The rate of evaporation of a liquid is affected by a number of factors, including temperature, surface area, and airflow. Airflow is the movement of air across the surface of a liquid. The greater the airflow, the more molecules of the liquid are able to escape into the gas phase, and the faster the evaporation rate.
- Role in evaporation: Airflow plays an important role in evaporation by removing water vapor from the surface of the liquid. This allows more molecules of the liquid to escape into the gas phase, and increases the evaporation rate.
- Examples: Airflow can be increased by using a fan or blower to blow air across the surface of the liquid. This is often done in industrial settings to speed up the evaporation process.
- Implications for "evaporation of normal propyl alcohol does heat evaporate faster than cold temp": Airflow is an important factor to consider when evaporating normal propyl alcohol. By increasing the airflow, it is possible to increase the evaporation rate and speed up the process.
In summary, airflow is an important factor that affects the rate of evaporation of normal propyl alcohol. By understanding the relationship between airflow and evaporation rate, it is possible to control the evaporation process and optimize the performance of products that use normal propyl alcohol.
Humidity
Humidity is a measure of the amount of water vapor in the air. The higher the humidity, the more water vapor is present in the air. Water vapor can slow down the evaporation rate of a liquid because it creates a barrier between the liquid and the air. This barrier makes it more difficult for molecules of the liquid to escape into the gas phase.
- Role in evaporation: Humidity plays an important role in evaporation by reducing the rate of evaporation. This is because water vapor in the air creates a barrier between the liquid and the air, making it more difficult for molecules of the liquid to escape into the gas phase.
- Examples: The effect of humidity on evaporation can be seen in everyday life. For example, clothes will dry more slowly on a humid day than on a dry day. This is because the water vapor in the air slows down the evaporation of the water from the clothes.
- Implications for "evaporation of normal propyl alcohol does heat evaporate faster than cold temp": Humidity is an important factor to consider when evaporating normal propyl alcohol. By understanding the relationship between humidity and evaporation rate, it is possible to control the evaporation process and optimize the performance of products that use normal propyl alcohol.
In summary, humidity is an important factor that affects the rate of evaporation of normal propyl alcohol. By understanding the relationship between humidity and evaporation rate, it is possible to control the evaporation process and optimize the performance of products that use normal propyl alcohol.
Pressure
The rate of evaporation of a liquid is affected by a number of factors, including temperature, surface area, airflow, and pressure. Pressure is the force exerted by a fluid (liquid or gas) per unit area. The higher the pressure, the more molecules of the liquid are forced together, and the less likely they are to escape into the gas phase. This means that the evaporation rate is slower at higher pressures.
The relationship between pressure and evaporation rate is important in a number of industrial and commercial applications. For example, in the production of paints and coatings, the pressure of the air is controlled to achieve the desired drying time. By understanding the relationship between pressure and evaporation rate, it is possible to control the drying process and optimize the performance of these products.
In the case of normal propyl alcohol, the evaporation rate is slower at higher pressures. This is because the molecules of normal propyl alcohol are forced together more tightly at higher pressures, making it less likely that they will escape into the gas phase. This means that the evaporation of normal propyl alcohol will take longer at higher pressures.
The effect of pressure on the evaporation rate of normal propyl alcohol is an important factor to consider in many industrial and commercial applications. By understanding the relationship between pressure and evaporation rate, it is possible to control the evaporation process and optimize the performance of products that use normal propyl alcohol.
FAQs on Evaporation of Normal Propyl Alcohol
This section provides answers to frequently asked questions (FAQs) about the evaporation of normal propyl alcohol, addressing common concerns and misconceptions.
Question 1: Does heat make normal propyl alcohol evaporate faster than cold temperatures?
Answer: Yes, the evaporation rate of normal propyl alcohol is directly proportional to temperature. Higher temperatures provide more energy for molecules to escape from the liquid and enter the gas phase, resulting in a faster evaporation rate.
Question 2: How does surface area affect the evaporation rate of normal propyl alcohol?
Answer: The evaporation rate of normal propyl alcohol increases with increasing surface area. A larger surface area allows for more molecules to be exposed to the air, facilitating their escape into the gas phase.
Question 3: What is the role of airflow in the evaporation of normal propyl alcohol?
Answer: Airflow enhances the evaporation rate of normal propyl alcohol. Moving air removes water vapor from the liquid's surface, creating a concentration gradient that promotes the diffusion of more molecules into the gas phase.
Question 4: How does humidity influence the evaporation rate of normal propyl alcohol?
Answer: Humidity has an inverse relationship with the evaporation rate of normal propyl alcohol. Higher humidity levels reduce the evaporation rate because water vapor in the air creates a barrier that hinders the escape of molecules from the liquid.
Question 5: What is the effect of pressure on the evaporation rate of normal propyl alcohol?
Answer: Pressure exhibits an inverse relationship with the evaporation rate of normal propyl alcohol. Higher pressure reduces the evaporation rate by compressing the molecules of the liquid, making it more difficult for them to escape into the gas phase.
Question 6: What are some practical applications of understanding the evaporation rate of normal propyl alcohol?
Answer: Understanding the evaporation rate of normal propyl alcohol is essential in various industrial and commercial applications, such as the production of paints, coatings, and solvents, where controlling the evaporation process is crucial for achieving desired results.
In summary, the evaporation of normal propyl alcohol is influenced by several factors, including temperature, surface area, airflow, humidity, and pressure. Understanding the interplay of these factors is essential for optimizing evaporation processes in practical applications.
Transition to the next article section:
Conclusion
The evaporation of normal propyl alcohol is a complex process that is affected by a number of factors, including temperature, surface area, airflow, humidity, and pressure. Understanding the interplay of these factors is essential for optimizing evaporation processes in practical applications.
In general, the evaporation rate of normal propyl alcohol increases with increasing temperature, surface area, and airflow, while decreasing with increasing humidity and pressure. By understanding these relationships, it is possible to control the evaporation process and achieve desired results in a variety of industrial and commercial applications.
The Ultimate Guide To Drop Ceiling Minimum Clearance Requirements
How Treatable Are Parasites?
Definitive Guide To The "Sister Act 2" Cast

How Fast Does Alcohol Evaporate [At Room Temperature and When Boiling
How Long Does It Take for Water To Evaporate? Water Filter Market

why does alcohol evaporate faster than water solsarin