What is the significance of molar heat?
Molar heat, or molar specific heat capacity, is a physical quantity that measures the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin. It is an intensive property, meaning that it is independent of the amount of substance present.
Molar heat is an important property for understanding the thermal behavior of substances. It can be used to calculate the amount of heat required to heat or cool a substance, and to design thermal insulation systems. Molar heat is also used in chemical reactions to calculate the enthalpy change of a reaction.
The molar heat of a substance depends on its chemical composition, molecular structure, and temperature. In general, the molar heat of a substance increases with increasing temperature. This is because the higher the temperature, the more energy is required to increase the temperature of the substance.
Molar heat is a useful property for understanding the thermal behavior of substances and for designing thermal insulation systems.
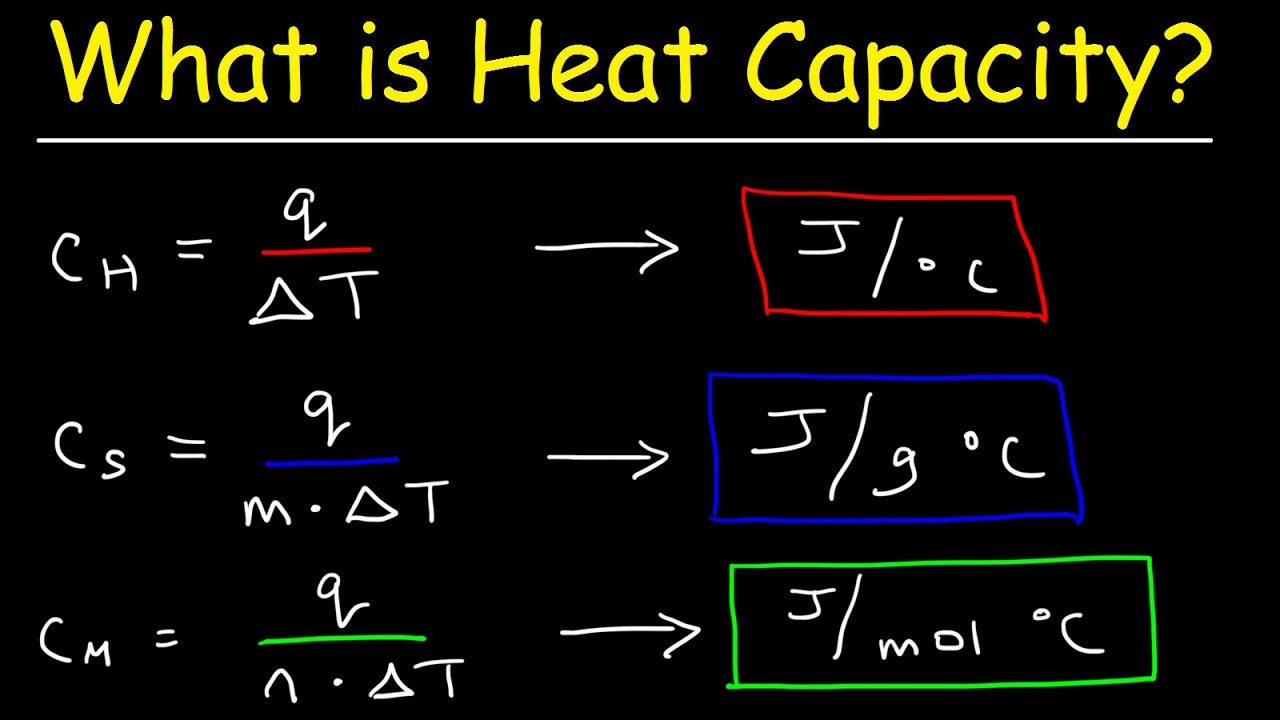
Molar heat
Molar heat, or molar specific heat capacity, is a physical quantity that measures the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin. It is an intensive property, meaning that it is independent of the amount of substance present.
- Definition: The amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin.
- Units: J/molK
- Importance: Used to calculate the amount of heat required to heat or cool a substance, and to design thermal insulation systems.
- Factors affecting molar heat: Chemical composition, molecular structure, and temperature.
- Applications: Thermal analysis, calorimetry, and chemical reactions.
- Examples: The molar heat of water is 75.3 J/molK, the molar heat of iron is 25.1 J/molK, and the molar heat of air is 29.1 J/molK.
Molar heat is a useful property for understanding the thermal behavior of substances and for designing thermal insulation systems. It is also used in chemical reactions to calculate the enthalpy change of a reaction.
Definition
This definition provides a concise and clear understanding of molar heat. It highlights that molar heat is a measure of the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin. This definition is essential for understanding the concept of molar heat and its applications.
- Components of molar heat: Molar heat is composed of two components: the amount of heat energy required to raise the temperature of the substance and the number of moles of the substance.
- Examples of molar heat: The molar heat of water is 75.3 J/molK, which means that 75.3 joules of heat energy are required to raise the temperature of one mole of water by one degree Celsius or one Kelvin.
- Implications of molar heat: Molar heat is an important property for understanding the thermal behavior of substances. It can be used to calculate the amount of heat required to heat or cool a substance, and to design thermal insulation systems.
In summary, the definition of molar heat provides a foundation for understanding its components, examples, and implications. This definition is crucial for comprehending the concept of molar heat and its applications in various fields.
Units
The units of molar heat are joules per mole Kelvin (J/molK). This means that molar heat is a measure of the amount of energy required to raise the temperature of one mole of a substance by one Kelvin.
- Joules: The joule is the SI unit of energy. It is defined as the amount of energy transferred or work done when a force of one newton is applied over a distance of one meter in the direction of the force.
- Moles: The mole is the SI unit of amount of substance. It is defined as the amount of substance that contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12.
- Kelvins: The kelvin is the SI unit of temperature. It is defined as 1/273.16 of the thermodynamic temperature of the triple point of water.
The units of molar heat are important because they provide a way to quantify the amount of energy required to raise the temperature of a substance. This information can be used to design thermal insulation systems, to calculate the amount of heat required to heat or cool a substance, and to understand the thermal behavior of substances.
Importance
Molar heat is an important property for understanding the thermal behavior of substances. It can be used to calculate the amount of heat required to heat or cool a substance, and to design thermal insulation systems.
- Calculating the amount of heat required to heat or cool a substance
Molar heat can be used to calculate the amount of heat required to raise the temperature of a substance by a certain amount. This information is useful for a variety of applications, such as designing heating and cooling systems, and calculating the energy consumption of appliances.
- Designing thermal insulation systems
Molar heat can be used to design thermal insulation systems that minimize heat loss or gain. This information is useful for a variety of applications, such as designing buildings, clothing, and food packaging.
Molar heat is a versatile property that has a wide range of applications in the field of thermal engineering. It is an essential property for understanding the thermal behavior of substances and for designing thermal insulation systems.
Factors affecting molar heat
The molar heat of a substance is affected by several factors, including its chemical composition, molecular structure, and temperature. These factors can have a significant impact on the amount of heat required to raise the temperature of a substance by one degree Celsius or one Kelvin.
Chemical composition
The chemical composition of a substance is one of the most important factors affecting its molar heat. Substances with different chemical compositions have different molar heats. For example, the molar heat of water is 75.3 J/molK, while the molar heat of iron is 25.1 J/molK.
Molecular structure
The molecular structure of a substance can also affect its molar heat. Substances with different molecular structures have different molar heats. For example, the molar heat of diamond is 5.0 J/molK, while the molar heat of graphite is 8.5 J/molK.
Temperature
The temperature of a substance can also affect its molar heat. In general, the molar heat of a substance increases with increasing temperature. This is because the higher the temperature, the more energy is required to increase the temperature of the substance.
Understanding the factors that affect molar heat is important for a variety of applications. For example, this understanding can be used to design materials with specific thermal properties, to develop new energy-efficient technologies, and to improve the efficiency of industrial processes.
Applications
Molar heat is a key property used in various applications, including thermal analysis, calorimetry, and chemical reactions.
Thermal analysis is a technique used to study the thermal properties of materials. Molar heat is an important parameter in thermal analysis, as it can be used to determine the specific heat capacity of a material. This information can be used to design materials with specific thermal properties, such as high thermal conductivity or low thermal expansion.
Calorimetry is a technique used to measure the amount of heat released or absorbed by a chemical reaction. Molar heat is an important parameter in calorimetry, as it can be used to determine the enthalpy change of a reaction. This information can be used to understand the thermodynamics of a reaction and to design chemical processes.
Chemical reactions involve the rearrangement of atoms and molecules, which can result in the release or absorption of heat. Molar heat is an important parameter in chemical reactions, as it can be used to calculate the amount of heat released or absorbed by a reaction. This information can be used to design chemical processes and to predict the products of a reaction.
Understanding the connection between molar heat and these applications is important for a variety of reasons. For example, this understanding can be used to design materials with specific thermal properties, to develop new energy-efficient technologies, and to improve the efficiency of industrial processes.
Examples
These examples illustrate the concept of molar heat and its variation across different substances. The molar heat of a substance is a measure of the amount of heat energy required to raise the temperature of one mole of that substance by one Kelvin. The examples provided show that the molar heat of water is significantly higher than that of iron and air. This difference is due to the differences in the molecular structure and bonding of these substances.
The molar heat of a substance is an important property that can be used to understand the thermal behavior of that substance. For example, the high molar heat of water makes it a good heat sink, meaning that it can absorb a large amount of heat without a significant increase in temperature. This property is important for many applications, such as cooling systems and heat exchangers.
Understanding the concept of molar heat and its variation across different substances is essential for a variety of applications in fields such as chemistry, materials science, and engineering.
Frequently Asked Questions about Molar Heat
This section addresses common questions and misconceptions about molar heat, providing concise and informative answers.
Question 1: What is molar heat?
Answer: Molar heat, also known as molar specific heat capacity, is the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin.
Question 2: What are the units of molar heat?
Answer: The SI unit of molar heat is joules per mole Kelvin (J/molK).
Question 3: What factors affect molar heat?
Answer: Molar heat is primarily affected by the chemical composition, molecular structure, and temperature of the substance.
Question 4: What are some applications of molar heat?
Answer: Molar heat is used in thermal analysis, calorimetry, chemical reactions, and various industrial processes.
Question 5: How does molar heat differ from specific heat?
Answer: Specific heat is the amount of heat energy required to raise the temperature of one gram of a substance by one degree Celsius or one Kelvin, while molar heat is the amount of heat energy required to raise the temperature of one mole of a substance by one degree Celsius or one Kelvin.
Question 6: What is the molar heat of water?
Answer: The molar heat of water is 75.3 J/molK.
Summary: Molar heat is a fundamental property of substances that quantifies the amount of heat required to raise their temperature. Understanding molar heat is essential for various applications in chemistry, physics, and engineering.
Transition to the next article section:
Conclusion
Molar heat, a crucial property of substances, quantifies the heat energy required to elevate their temperature. Throughout this exploration, we have gained insights into its definition, units, influencing factors, applications, and examples.
Understanding molar heat empowers us to comprehend the thermal behavior of substances, design efficient thermal systems, and optimize chemical reactions. Its significance extends to diverse fields, including materials science, engineering, and environmental studies. As we continue to delve into the realm of thermal properties, molar heat will undoubtedly remain a cornerstone of our knowledge.
Can You Plant Daffodil Bulbs In January For A Stunning Spring Display?
The Interwoven Threads: Unveiling The Striking Similarities Between Contemporary And Modern Arts
Today's Utah Valley Air Quality: Is It A Burn Day?

Molar Specific HeatSpecific heat, And Types

Molar Heat Capacity Problems Physics YouTube
What Is The Difference Between Specific Heat Capacity, Heat Capacity